EMA recommends approval of Imvanex for prevention of monkeypox
23 July 2022
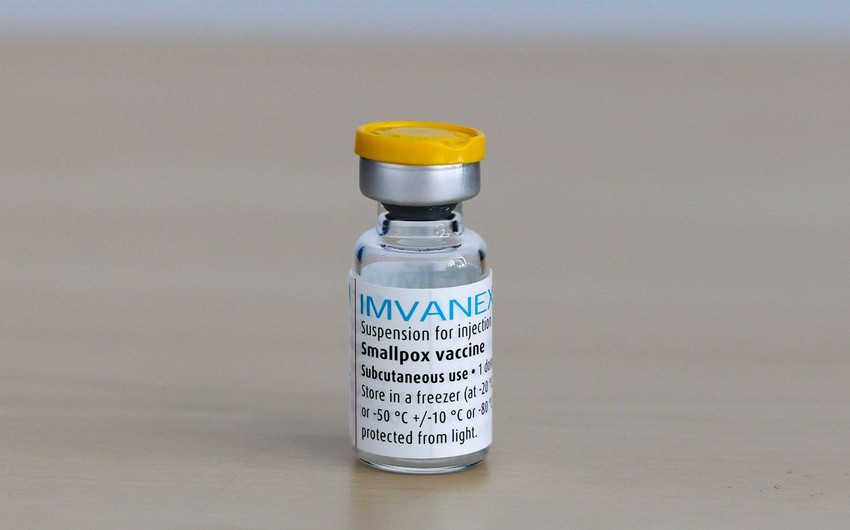
EMA’s human medicines committee (CHMP) has recommended extending the indication of the smallpox vaccine Imvanex to include protecting adults from monkeypox disease.
Report informs, citing EMA's website, that the medicine has been approved in the EU since 2013 for the prevention of smallpox. It contains an attenuated (weakened) form of the vaccinia virus called ‘modified vaccinia virus Ankara’, which is related to the smallpox virus. It was also considered a potential vaccine for monkeypox because of the similarity between the monkeypox virus and the smallpox virus. The marketing authorisation holder is Bavarian Nordic A/S.
The CHMP based their recommendation on data from several animal studies which showed protection against the monkeypox virus in non-human primates vaccinated with Imvanex. The safety profile of the medicine is favourable, with mild to moderate side effects, and the CHMP concluded that the medicine’s benefits are greater than the risks.
https://report.az/en/health/ema-recommends-approval-of-imvanex-for-prevention-of-monkeypox/
Report informs, citing EMA's website, that the medicine has been approved in the EU since 2013 for the prevention of smallpox. It contains an attenuated (weakened) form of the vaccinia virus called ‘modified vaccinia virus Ankara’, which is related to the smallpox virus. It was also considered a potential vaccine for monkeypox because of the similarity between the monkeypox virus and the smallpox virus. The marketing authorisation holder is Bavarian Nordic A/S.
The CHMP based their recommendation on data from several animal studies which showed protection against the monkeypox virus in non-human primates vaccinated with Imvanex. The safety profile of the medicine is favourable, with mild to moderate side effects, and the CHMP concluded that the medicine’s benefits are greater than the risks.
https://report.az/en/health/ema-recommends-approval-of-imvanex-for-prevention-of-monkeypox/